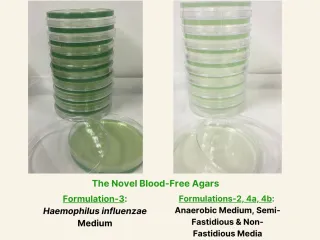
Description
Blood - based media are well established and routinely utilized bacteriological media. Animals like sheep and horses are specially bred for the purpose of manufacturing blood agar plates. This endeavour is expensive, labour intensive, associated with a high carbon footprint and animal welfare concerns. It also poses a health risk to laboratory workers due to the potential for transmission of blood – borne pathogens from the source animal blood / animal extract to the laboratory operator. Affiliated with the International Medical University (Bukit Jalil, Kuala Lumpur, Malaysia), I have developed four varieties of NOVEL bacteriological media, that are COMPLETELY BLOOD FREE and ANIMAL EXTRACT FREE, for the cultivation of both hemophilic (blood – loving) and non – hemophilic (non - blood – loving) bacteria. The NOVEL Agars support good to excellent growth of a variety of bacterial pathogens. In addition, they have a SUPERIOR health, safety, economic, ethical and environmental profile in comparison to blood – based agars. As blood agar plates are used routinely in diagnostic and research laboratories worldwide, the market volume for the novel, blood – free and animal – extract free agars targeted to replace blood agars in the laboratory is expected to be commensurately huge.
Highlights
COMMERCIALISATION STATUS: Patent pending. We have completed circa 60% of research and development associated with TECHNOLOGY READINESS LEVEL-6 (TRL-6)**. DESCRIPTION OF THE INVENTION: Affiliated with the International Medical University (Bukit Jalil, Kuala Lumpur, Malaysia), I have developed four varieties of NOVEL bacteriological media, that are COMPLETELY BLOOD – FREE and ANIMAL – EXTRACT FREE, for the cultivation of both hemophilic (blood – loving) and non – hemophilic (non - blood – loving) bacteria. NEED FOR THE INVENTION: Blood based media are well established and routinely utilized bacteriological media for the cultivation of a variety of pathogens. In addition, when the identity of a microorganism is completely unknown, as is often the case in a diagnostic microbiology laboratory, blood agar is the medium of choice for isolation of animal pathogens. Animals like sheep, rabbits, cattle, goats and horses are specially bred for the purpose of manufacturing blood agar plates for microbiological culture and experimentation. The manufacture of blood agar incurs high expense and has a high carbon footprint. Animal husbandry is a major contributor to global methane emissions whilst massive deforestation to cultivate animal feed is a related environmental issue of concern. Animal blood harvesting is a tedious process associated with animal welfare concerns and the issue of animal autonomy (as associated with animal ethics). Most importantly, the use of animal blood and animal extracts in microbiology poses a health risk to laboratory workers due to the potential for transmission of blood – borne pathogens from the source animal blood / animal extract to the laboratory operator. Underpinning the latter health and safety risk, labels on bottled dehydrated and animal – extract containing powdered microbiological media clearly state that the manufacturer cannot guarantee if the extract is pathogen-free. Due to the rising consciousness towards animal sentience and welfare, coupled with the widely acknowledged human health, safety, economic and environmental concerns associated with the laboratory use of animal blood (and other animal extracts), it has become expedient that alternatives to animal blood based microbiological media be developed. COMPARATIVE PERFORMANCE OF THE NOVEL, BLOOD – FREE & ANIMAL – EXTRACT FREE AGARS VERSUS BLOOD – BASED AGARS: The NOVEL Agars support good to excellent growth of a variety of hemophilic (blood – loving) and non – hemophilic (non – blood – loving) bacterial pathogens. In addition, they have a SUPERIOR health, safety, economic, ethical and environmental profile in comparison to blood – based agars. PREDICTED MARKET VOLUME: The global market volume is predicted to be huge as blood agar plates are used routinely in diagnostic and research laboratories worldwide. As such, given a grace period for conditioning of the market, the novel, blood – free and animal – extract free agars that are targeted to replace blood agars in the laboratory, are expected to have a commensurately huge market volume. * A HEMOPHILIC bacterium has a partial or strict requirement for blood for its growth ** NASA/SP - 20076105: A scale for measuring the maturity of a technology
Contact Person/Inventor
| Name | Contact Phone | |
|---|---|---|
| Fatimah Binti Muhammad | FatimahMuhammad@imu.edu.my | 60129497196 |
MRDCS
Award
| Award Title | Award Achievement | Award Year Received |
|---|---|---|
| International Innovation Awards | MTE 2023 | 2023 |
Event Participation
| Event Name | Event Year |
|---|---|
| Malaysia Technology Expo | 2023 |








Comment